Plant-based food products are emerging rapidly, and plant proteins are crucial in creating these products. First, these proteins need to be extracted from plant crops. The extraction is generally performed with a resource-demanding and intensive process with many process steps. The result is a protein ingredient with high purity (>80%).1 A major drawback of such a process is the loss of a protein group, namely the albumins. This article will explain why albumins are lost in the current process, how they can be retained and their high potential as a food ingredient.
This article was written by Dr Jack Yang, a Development Specialist at FrieslandCampina in the Netherlands.
Conventional protein extraction — separating albumins and globulins
The conventional (and most used) method separates the albumins and globulins. Plant proteins are classified with the Osborne classification, based on solubility. Albumin proteins are soluble in water and in a pH range from 2 to 10.2 Globulin proteins are soluble in dilute salt solutions and often have an isoelectric point between 4 and 5. At these pH values, the globulins have a net zero charge, leading to aggregation, and extremely low solubility. This effect is currently used to obtain a pure globulin-rich protein extract, while discarding the albumins.
Conventional protein extraction, known as the alkaline–-acid precipitation method, starts with pre-processing (dehulling, defatting and milling). The resulting flour is then dispersed in water, and the pH is adjusted to alkaline values (pH 8 – 13).3A high pH leads to a higher solubility of the globulins, which increases the protein yield of the processes. Afterwards, the insoluble components (often starch and fibres) are removed by centrifugation or decanting. The soluble fraction contains globulins, albumins and other solutes. These non-protein solutes contain sugars, phenols, and minerals. Some components act as off-flavours or anti-nutritional factors, which are removed using isoelectric point precipitation. Globulins are precipitated, while the non-protein solutes and the albumins remain soluble. These two streams are separated using a second centrifugation/decanting step. The result is a globulin-rich pellet that is further processed into a protein isolate (>80%), while the supernatant contains non-protein solutes and albumins. This supernatant is generally discarded as wastewater.
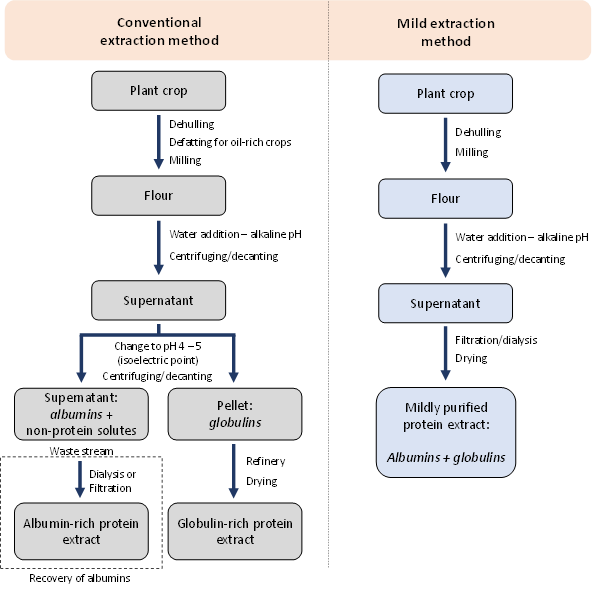
Figure 1. Schematic overview of conventional and mild extraction methods.
Recovering and retaining albumin proteins
The utilisation of the albumins in this waste stream should be considered, as plant seeds can contain between 10 – 25% albumins.4 This suggests the loss of up to 25% of the plant proteins using the conventional extraction method. One way to recover albumins is by removing the non-protein solutes in the waste stream using dialysis or ultrafiltration methods. An example is shown for pea albumins, which were obtained with dialysis with a 2 kDa cut-off to obtain an albumin-rich extract with 52% protein. This protein content can be increased by optimising the dialysis/filtration method by, for instance, using a larger pore size.
Another option is the retention of albumins in the process by omitting the isoelectric point step, known as mild protein extraction. This allows the co-extraction of albumins and globulins, but the non-protein components are, of course, still present. Here, dialysis/filtration can play a major role, leading to mildly purified protein extracts with protein contents of >80%.5
FSTA contains a wealth of reliable, interdisciplinary, food-focused information, making it a great tool for researching published science on the extraction of albumins from plant sources. For example, approximately 500 documents focusing on the extraction of plant albumins have been indexed in our FSTA database. These are sourced from over 170 journals published by 67 organizations based in 32 countries.
Read more facts about FSTA content on this topic, including key journals and FSTA descriptors.
Techno-functionality of albumin proteins
A major difference between albumins and globulins is their solubility. Often, globulins possess poor solubility at the typical food pH ranges (4 – 7), while albumins are highly soluble. This high solubility is, for instance, beneficial in foaming properties. In several studies, foaming properties were studied of albumin and globulin proteins from Bambara groundnut, mung bean and yellow pea.4,6,7Albumin protein solutions could create foam with volumes and stability as high as dairy proteins, and even showed higher stability compared to egg proteins. Globulin protein solutions performed poorly, with about five times lower foam volumes and 5 to 10 times lower foam stability compared to the albumin proteins. Protein extracts containing both albumins and globulins also had improved foaming properties compared to pure globulin extracts.7
In emulsion stabilisation, albumins and globulins from rapeseed seem to have a synergistic effect. Rapeseed albumin stabilises the oil droplet, and the globulin forms a second layer around the droplet to increase flocculation stability.8 An albumin and globulin protein mixture also gave higher gel strength than pure globulin gels.9 This could be related to the albumin protein but also due to more native globulin. Mild extraction will also give more native globulin proteins, increasing gelling properties. Albumins also seem more stable against heating, which is reflected in higher denaturation temperatures, as shown for pea and rapeseed.9,10 The result of aggregate formation by globulins upon extensive heating and spray drying, while albumin remained soluble.
Outlook and challenges
Albumins are a neglected protein stream, while they possess great techno-functionality. The proteins can be recovered from the waste stream or co-extracted with globulin proteins. Plant protein ingredient manufacturers should consider utilising these future food ingredients, especially due to their high solubility, superior foaming properties and high thermal stability. Also, academic attention is required to discover new functionalities and address several challenges. Albumin proteins have not received as much attention as globulins, especially in terms of nutrition. Digestion, allergenicity and safety of these novel ingredients should definitely be carefully evaluated. Afterwards, albumins can be utilised as a high-end functional ingredient in our future plant-based foods.
References
- Yang, J.; Sagis, L. M. C. Interfacial Behavior of Plant Proteins – Novel Sources and Extraction Methods. Curr. Opin. Colloid Interface Sci. 2021, 56, 101499. https://doi.org/10.1016/j.cocis.2021.101499.
- Gonzalez-Perez, S.; Vereijken, J. M.; van Koningsveld, G. A.; Gruppen, H.; Voragen, A. G. J. Physicochemical Properties of 2S Albumins and the Corresponding Protein Isolate from Sunflower (Helianthus Annuus). Food Chem. Toxicol. 2005, 70 (1), C98–C103.
- Sari, Y. W.; Mulder, W. J.; Sanders, J. P. M.; Bruins, M. E. Towards Plant Protein Refinery: Review on Protein Extraction Using Alkali and Potential Enzymatic Assistance. Biotechnol. J. 2015, 10 (8), 1138–1157. https://doi.org/10.1002/biot.201400569.
- Yang, J.; Kornet, R.; Diedericks, C. F.; Yang, Q.; Berton-Carabin, C. C.; Nikiforidis, C. V.; Venema, P.; van der Linden, E.; Sagis, L. M. C. Rethinking Plant Protein Extraction: Albumin — from Side Stream to an Excellent Foaming Ingredient. Food Struct. 2022, 31, 100254. https://doi.org/10.1016/j.foostr.2022.100254.
- Kornet, R.; Shek, C.; Venema, P.; Jan van der Goot, A.; Meinders, M.; van der Linden, E. Substitution of Whey Protein by Pea Protein Is Facilitated by Specific Fractionation Routes. Food Hydrocoll. 2021, 117, 106691. https://doi.org/10.1016/j.foodhyd.2021.106691.
- Kornet, R.; Yang, J.; Venema, P.; van der Linden, E.; Sagis, L. Optimizing Pea Protein Fractionation to Yield Protein Fractions with a High Foaming and Emulsifying Capacity. Food Hydrocoll. 2022, 126, 107456. https://doi.org/10.1016/j.foodhyd.2021.107456.
- Yang, J.; de Wit, A.; Diedericks, C. F.; Venema, P.; van der Linden, E.; Sagis, L. M. C. Foaming and Emulsifying Properties of Extensively and Mildly Extracted Bambara Groundnut Proteins: A Comparison of Legumin, Vicilin and Albumin Protein. Food Hydrocoll. 2022, 123, 107190. https://doi.org/10.1016/j.foodhyd.2021.107190.
- Ntone, E.; Wesel, T. Van; Sagis, L. M. C.; Meinders, M.; Bitter, J. H.; Nikiforidis, C. V. Adsorption of Rapeseed Proteins at Oil / Water Interfaces . Janus-like Napins Dominate the Interface. J. Colloid Interface Sci. 2021, 583, 459–469. https://doi.org/10.1016/j.jcis.2020.09.039.
- Kornet, R.; Veenemans, J.; Venema, P.; van der Goot, A. J.; Meinders, M.; Sagis, L.; van der Linden, E. Less Is More: Limited Fractionation Yields Stronger Gels for Pea Proteins. Food Hydrocoll. 2021, 112, 106285. https://doi.org/10.1016/j.foodhyd.2020.106285.
- Yang, J.; Faber, I.; Berton-Carabin, C. C.; Nikiforidis, C. V.; van der Linden, E.; Sagis, L. M. C. Foams and Air-Water Interfaces Stabilised by Mildly Purified Rapeseed Proteins after Defatting. Food Hydrocoll. 2020, 112, 106270. https://doi.org/10.1016/j.foodhyd.2020.106270.
Researching the extraction of albumins from plant sources
The extraction of albumins from plant sources is a core topic for FSTA. The IFIS Science team will select and index all articles relevant to this field that are published in the high-quality sources currently being monitored for FSTA content. Approx. 500 documents reaching back to 1968 have been indexed focusing on the extraction of plant albumins. These are sourced from >170 journals published by 67 organizations based in 32 countries.
Examples of recent records include:
-
Functional, thermal and structural properties of fractionated protein from waste banana peel. Food Chemistry: X 13 100205. FSTA: 2023-01-Jg0540
-
Selective extraction of napins: process optimization and impact on structural and functional properties.Food Hydrocolloids 122 107105. FSTA: 2022-01-Ne0017
-
Pine nuts (Pinus pinea L.) as a potential novel plant-based source of functional protein isolates: optimization of alkali extraction conditions, evaluation of functional properties, and biochemical characterization.Journal of Food Processing and Preservation 46 (4) e16471. FSTA: 2022-07-Js10248
-
Preparation and identification of an antioxidant peptide from wheat embryo albumin and characterization of its Maillard reaction products. Journal of Food Science 87 (6) 2549-2562. FSTA: 2022-10-Mj6065
-
Chemical and functional characterization of major protein fractions extracted from nontoxic Jatropha curcas byproduct meals. Journal of the American Oil Chemists' Society 99 (6) 511-523. FSTA: 2022-10-Js13751


